BOTOX® Cosmetic
Botox Cosmetic, Dermal Fillers, Botox Specials, Allergan Products
What is Botox® Cosmetic?
 BOTOX® Cosmetic is a prescription medicine that is injected into muscles and used to improve the look of moderate-to-severe frown lines between the brows in people 18 to 65 years of age for a short period of time (temporary).
BOTOX® Cosmetic is a prescription medicine that is injected into muscles and used to improve the look of moderate-to-severe frown lines between the brows in people 18 to 65 years of age for a short period of time (temporary).
BOTOX® Cosmetic is administered by a healthcare professional as a simple, nonsurgical treatment that is injected directly into the muscles between the brows. It works by blocking nerve impulses to the injected muscles. This reduces muscle activity that causes moderate to severe lines to form between the brows.
To schedule an appointment for a Free Skin Consultation, call us at one of our locations listed below.
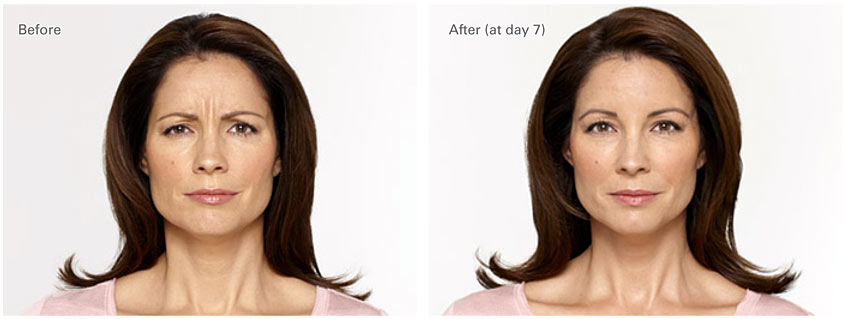
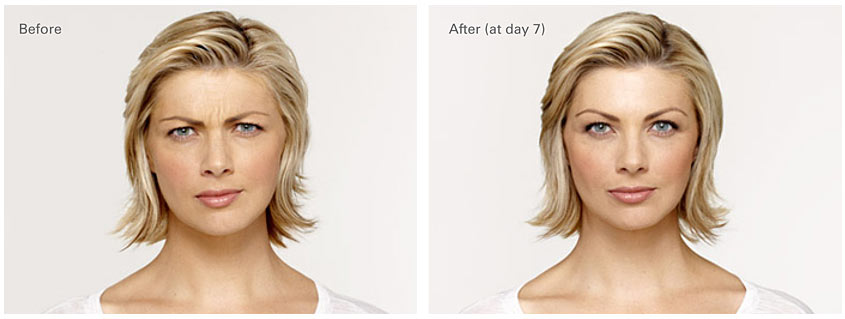
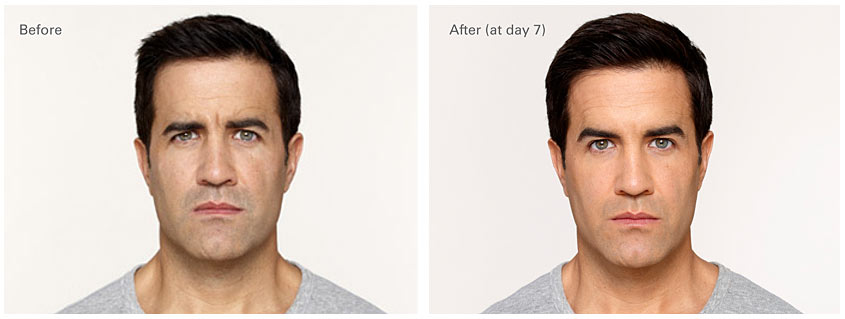
Before and After Unretouched Photos
Alecia
Amy
Jonathan
To learn more, visit: Botoxcosmetic.com
Results may vary by individual. Results shown are not a guarantee of outcome.
To schedule an appointment for a Free Skin Consultation, call us at one of our locations listed below.
Safety Information
Indication
BOTOX® Cosmetic is indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity in patients 18 to 65 years of age.
IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING
Distant Spread of Toxin Effect
Postmarketing reports indicate that the effects of BOTOX® Cosmetic and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening, and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity, but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses, including spasticity in children and adults, and in approved indications, cases of spread of effect have occurred at doses comparable to those used to treat cervical dystonia and at lower doses.
BOTOX® Cosmetic (onabotulinumtoxinA)
IMPORTANT SAFETY INFORMATION (continued)
CONTRAINDICATIONS
BOTOX® Cosmetic is contraindicated in the presence of infection at the proposed injection site(s) and in individuals with known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation.
WARNINGS
The recommended dosage and frequency of administration for BOTOX® Cosmetic should not be exceeded. Risks resulting from administration at higher dosages are not known.
Lack of Interchangeability Between Botulinum Toxin Products
The potency Units of BOTOX® Cosmetic are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, Units of biological activity of BOTOX® Cosmetic cannot be compared to or converted into Units of any other botulinum toxin products assessed with any other specific assay method.
Spread of Toxin Effect
Please refer to Boxed Warning for Distant Spread of Toxin Effect. No definitive, serious adverse event reports of distant spread of toxin effect associated with dermatologic use of BOTOX® Cosmetic at the labeled dose of 20 Units (for glabellar lines) have been reported.
Hypersensitivity Reactions
Serious and/or immediate hypersensitivity reactions have been reported. These reactions include anaphylaxis, urticaria, soft-tissue edema, and dyspnea. If such reactions occur, further injection of BOTOX® Cosmetic should be discontinued and appropriate medical therapy immediately instituted. One fatal case of anaphylaxis has been reported in which lidocaine was used as the diluent and, consequently, the causal agent cannot be reliably determined.
Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junctional disorders (eg, myasthenia gravis or Lambert-Eaton syndrome) should be monitored particularly closely when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including severe dysphagia and respiratory compromise from typical doses of BOTOX® Cosmetic.
Human Albumin
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin.
PRECAUTIONS
Caution should be used when BOTOX® Cosmetic treatment is used in patients who have an inflammatory skin problem at the injection site, marked facial asymmetry, ptosis, excessive dermatochalasis, deep dermal scarring, thick sebaceous skin, or the inability to substantially lessen glabellar lines by physically spreading them apart.
Information for Patients
Patients should be counseled that if loss of strength, muscle weakness, or impaired vision occur, they should avoid driving a car or engaging in other potentially hazardous activities.
Drug Interactions
Co-administration of BOTOX® Cosmetic and aminoglycosides or other agents interfering with neuromuscular transmission (eg, curare-like nondepolarizing blockers, lincosamides, polymyxins, quinidine, magnesium sulfate, anticholinesterases, succinylcholine chloride) should only be performed with caution as the effect of the toxin may be potentiated.
The effect of administering different botulinum neurotoxin serotypes at the same time or within several months of each other is unknown. Excessive neuromuscular weakness may be exacerbated by administration of another botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin.
Pregnancy
Administration of BOTOX® Cosmetic is not recommended during pregnancy. There are no adequate and well-controlled studies of BOTOX® Cosmetic in pregnant women.
Nursing Mothers
It is not known whether BOTOX® Cosmetic is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when BOTOX® Cosmetic is administered to a nursing woman.
ADVERSE REACTIONS
General
The most serious adverse events reported after treatment with botulinum toxin include spontaneous reports of death, sometimes associated with anaphylaxis, dysphagia, pneumonia, and/or other significant debility.
There have also been reports of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes. Some of these patients had risk factors including pre-existing cardiovascular disease.
The most frequently reported adverse events following injection of BOTOX® Cosmetic include blepharoptosis and nausea.
Please see BOTOX® Cosmetic full Prescribing Information including Boxed Warning and Medication Guide.
©2011 Allergan, Inc., Irvine, CA 92612 ® and ™ marks owned by Allergan, Inc.
KP, Dry Skin, and Glytone Body Therapy – Letrice Eagle, ST
Skin gets dry in the fall and winter due to low humidity, both outside and inside the house. Exposure to dry wind, hot shower water or drying soaps...
How Do You Choose the Right Sunscreen? Dr. Erin Reid
Using sunscreen every day reduces your risk of skin cancer, which is the most common type of cancer in the United States. Sunscreen also prevents...
Facial Aging Goes Beyond Wrinkles – Dermatology Associates
Aging - it's a fact of life. You can't help getting older, but you can take control of how you age. There are many factors that cause our skin...